I would like some information regarding how the iPhone battery works so I can better understand my iPhone 6.
My device:
I am on an iPhone 6, 64 GB model in Space Grey, that was purchased new from Verizon on January 14 of this year.
My iPhone management habits:
* I never manually close apps unless an app is misbehaving
* Once per month I allow my iPhone battery to run down until the phone powers itself off. I then charge it to 100% before using it again.
* The main app background refresh is on but toggled off for individual apps
* Screen timeout is set to 2 minutes
* Screen brightness is set to half with Auto-Brightness toggled on
The fact that I average 2 days between charges with moderate use tells me that these habits are beneficial. I have followed these habits on iPhones since 2012 and have always enjoyed long battery life on iPhones.
Symptom:
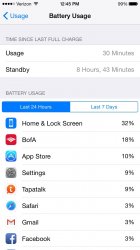
When I charge the phone to 100% and immediately begin using it, the phone stays on 100% for a short time. However, as you can see from the included screenshot, when I charge the phone to 100% and leave it on the charger for a couple hours after the 100% mark has been reached, the phone stays on 100% for more than 6 hours.
My query:
What exactly is happening, when I leave the phone on the charger for a couple hours after the 100% mark has been reached, that causes the apparent extended battery life? Am I shortening the overall lifespan of the battery by leaving it on the charger for hours after the 100% mark.
Can someone who understands these batteries give some insight?
My device:
I am on an iPhone 6, 64 GB model in Space Grey, that was purchased new from Verizon on January 14 of this year.
My iPhone management habits:
* I never manually close apps unless an app is misbehaving
* Once per month I allow my iPhone battery to run down until the phone powers itself off. I then charge it to 100% before using it again.
* The main app background refresh is on but toggled off for individual apps
* Screen timeout is set to 2 minutes
* Screen brightness is set to half with Auto-Brightness toggled on
The fact that I average 2 days between charges with moderate use tells me that these habits are beneficial. I have followed these habits on iPhones since 2012 and have always enjoyed long battery life on iPhones.
Symptom:
When I charge the phone to 100% and immediately begin using it, the phone stays on 100% for a short time. However, as you can see from the included screenshot, when I charge the phone to 100% and leave it on the charger for a couple hours after the 100% mark has been reached, the phone stays on 100% for more than 6 hours.
My query:
What exactly is happening, when I leave the phone on the charger for a couple hours after the 100% mark has been reached, that causes the apparent extended battery life? Am I shortening the overall lifespan of the battery by leaving it on the charger for hours after the 100% mark.
Can someone who understands these batteries give some insight?